Ammonium chloride industrial grade
-
Post Date:
Mar 17,2015 -
Expiry Date:
Mar 16,2016 -
Detailed Description:
Cas No. :12125-02-9 Quantity: 100Metric Tons
Specs:purity:99.5% , white crystal powder
Price:100 USD Metric Tons
Payment Method: T/T,L/C at sight
Ammonium chloride can be made by the chemical reaction , that is NH3+ HCI=NH4CI, which can get the cubic crystal or white powder, and that is the rudiment of ammonium chloride. We used this as material and deduce the scaps,Ammonium chloride can be widely used in medicine, feed additive ,smelting, chemistry analysis.we expert in producing the high purity,quality and precision ammonium chloride,
Technical index:
INDEX GB2946-92 RESULT AMMONIUM CHLORIDE % ≥ (NH4CL) 99.3 99.58 MOISTURE % ≤ 0.7 0.3 RESIDUE ON IGNITION % ≤ 0.4 0.2 HEAVY METAL(Pb) % ≤ 0.0005 0.00045 SULPHATE % ≤ 0.02 0.018 Fe % ≤ 0.001 0.0005 PH value 4-5.8 5.6 INDEX GB2946-92 RESULT AMMONIUM CHLORIDE % ≥ (NH4CL) 99.3 99.58 MOISTURE % ≤ 0.7 0.3 RESIDUE ON IGNITION % ≤ 0.4 0.2 HEAVY METAL(Pb) % ≤ 0.0005 0.00045 SULPHATE % ≤ 0.02 0.018 Fe % ≤ 0.001 0.0005 PH value 4-5.8 5.6 -
CAS Registry Number:
12125-02-9 -
Synonyms:
;Salmiac;Amchlor;ammonium muriate;Darammon;Sal ammoniac;Salammonite; -
Molecular Formula:
NH4Cl -
Molecular Weight:
53.4916 -
Molecular Structure:
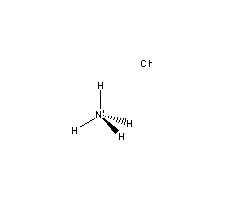
-
Hazard Symbols:
Xn:Harmful;
-
Risk Codes:
R22:;
R36:;
-
Safety Description:
S22:;
-
Company:
Zhuzhou JiangHai Environment Protection Co.Ltd -
Contact:
Tang -
Tel:
0731-22429291 -
Fax:
0731-28630109 -
Email:
elieen@zzjhhb.cn