Bortezomib 179324-69-7
-
Post Date:
May 07,2019
-
Expiry Date:
Nov 03,2019
-
Detailed Description:
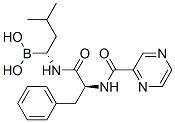
Cas No. :179324-69-7
Bortezomib is the first proteasome inhibitor to be approved by the US FDA for multiple myeloma, a blood cancer. A reversible inhibitor of the 26S proteasome-a barrel-shaped multiprotein particle found in the nucleus and cytosol of all eukaryotic cells. Targets the ubiquitin-proteasome pathway.
-
CAS Registry Number:
179324-69-7
-
Synonyms:
;VELCADE;dpba;[(1r)-3-methyl-1-[[(2s)-1-oxo-3-phenyl-2-[(pyrazinylcarbonyl)amino]propyl]amino]butyl]-boronic acid;VELCADE(BORTEZOMIB);Boronic acid, B-[(1R)-3-methyl-1-[[(2S)-1-oxo-3-phenyl-2-[(2-pyrazinylcarbonyl)amino]propyl]amino]butyl]-;Bortezomib for research;N-[(1S)-1-(dihydroxyboranyl)-3-methylbutyl]-Nalpha-(pyrazin-2-ylcarbonyl)-D-phenylalaninamide;N-[(1R)-1-(dihydroxyboranyl)-3-methylbutyl]-Nalpha-(pyrazin-2-ylcarbonyl)-L-phenylalaninamide;Bortizomib;
-
Molecular Formula:
C19H25BN4O4
-
Molecular Weight:
384.2372
-
Molecular Structure:
Inquiry
