ChissoS210
-
Post Date:
Apr 02,2025
-
Expiry Date:
Sep 29,2025
-
Detailed Description:
Cas No. :2768-02-7
Specs:98%
Payment Method: L/C,T/T,Western Union
CAS No.:2768-02-7
Silane coupling Agent KH-171
Vinyltrimethoxysiane
CAS No.:2768-02-7
Silane coupling Agent KH-171
Introduction:
KH-171 silane, vinyltrimethoxysiane, is used as a polymer modifier via grafting reactions. The resulting pendant trimethoxysilyl groups can function as moisture-activated crosslinking sites. The silane grafted polymer is processed as a thermoplastic and crosslinking occurs after fabrication of the finished article upon exposure to moisture.
Typical Physical Properties:
CAS No.: 2768-02-7
Molecular Weight: 148.2
Boiling Point<760mmHg>: 122
Flash Point: 28
Color and Appearance: Colorless transparent liquid Density<25/25>: 0.960-0.970
Refractive Index<25>: 1.3905+0.0005
Min. Purity: 98.0%
Applications:
1- Polymer Modification: KH-171 is used to modify polyethylene and other polymers by grafting its vinyl group to the polymer backbone using a radical initiator,such as peroxide. This provides a polymer with pendant trimethoxysilyl groups that may be used as moisture-activated crosslinking sites via hydrolysis of the alkoxy groups followed by condensation of the resulting silanols.
2- Crosslinking of Silane-Grafted Polymers: The reaction of Silane-grafted polyethylene to form a crosslinked or vulcanized polyethylene uses water to form the crosslinks. This technology is widely used around the world for commercial applications in wire and cable insulation, tubing, and other similar uses. The basic reaction sequence is as follows: polyethylene is reacted(grafted) with vinyltimethoxysilane, using a peroxide initiator, in an extruder. The grafted polyethylene is then formed into a finished product, such as cable j acketing, wire insulation, or pipe. The forming step is usually done by a second extrusion, during which a catalyst for the moisture-cure step is added. Finally, the formed article is exposed to moisture or hot water to cause hydrolysis of the Silane and condensation to form crosslinks via Si-O-Si bond formation.
3- Benefits of Crosslinking:
a) Higher maximum use temperature
b) Reduced deformation under load (creep)
c) Improved chemical resistance
d) Increased abrasion resistance
e) Improved impact strength
f) Memory characteristics (shrink film, tubing)
g) Improved impact strength
4- Advantages of Silane Crosslinking over Radiation or Peroxide Crosslinking
a) Low capital investment
b) Low operating (energy) costs
c) Higher productivity
d) Processing versatility
e) Thick, thin, or variable thicknesses possible
f) Complex shapes possible
g) Wilder processing latitude (control of premature crosslinking)
h) Useful with filled composites
i) Applicable to all polyethylene densities and copolymers.
-
CAS Registry Number:
2768-02-7
-
Synonyms:
;Ethenyltrimethoxysilan;Trimethoxy(vinyl)silane;Vinymethyltrimethoxysilane;Trimethoxysilyl)ethylene;Ethenyltrimethoxysilane;VTMO;Silane Coupling Agent A-171;Vinyl teimethoxy silane;Silane Coupling Agent Si-171;Vinyl Trimethoxy Silane;Vinyltrimethoxysilane;
-
Molecular Formula:
C5H12O3Si
-
Molecular Weight:
148.2325
-
Molecular Structure:
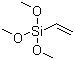
-
Hazard Symbols:
 Xi:Irritant;
Xi:Irritant;
-
Risk Codes:
R10:;
R36/37/38:;
-
Safety Description:
S16:;
S26:;
S37/39:;
Inquiry
Xi:Irritant;