Chondrotin Sulfate Injectable
-
Post Date:
Jan 17,2025
-
Expiry Date:
Jul 16,2025
-
Detailed Description:
Cas No. :9082-07-9
Specs:CP2010
Chondroitin Sulphate Injectable
1. Appearance:White to off white powder
2. Source:Terrestrial Cartilage
3. Purity: 98%
4. Standard: CP2010
Function of Chondroitin Sulphate Injectable
Compared with oral consumption,taking Chondrotin via injection takes effect quicker,has s shorter treatment cycle and gives a larger dosage pre time.It is used for treatment of nevous headache,neuralgin,joint pain,atherosclerosis and etc.
-
CAS Registry Number:
9082-07-9
-
Synonyms:
;Chondroitin Sulfate Sodium;chondroitin 4-sulfate sodium salt from bovine trachea;[(2R,3R,4R,5R,6R)-5-acetamido-4-[(2S,3S,4R,5R,6R)-6-carboxy-3,4,5-trihydroxy-tetrahydropyran-2-yl]oxy-6-hydroxy-2-(hydroxymethyl)tetrahydropyran-3-yl]oxysulfonylperoxysodium;CSA;Chondroitin Sulphate Sodium;Chondroitin sulfate A sodium salt;Chondroitin 6-sulfate sodium salt;Chondroitin Sulfate C Sodium Salt;Csc-Na;Shark Chondroitin Sulfate;Shark Chondroitin;Chondroitin-6-Sulfate Sodium Sult;
-
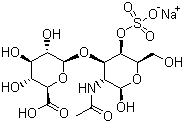
Molecular Formula:
C14H22NNaO16S
-
Molecular Weight:
515.3763
-
Molecular Structure:
Inquiry
