Diatrizoate sodium
-
Post Date:
Aug 14,2017
-
Expiry Date:
Aug 14,2018
-
Detailed Description:
Cas No. :737-31-5
Diatrizoate sodium
CAS: 737-31-5
MF: C11H8I3N2NaO4
MW: 635.9
EINECS: 212-004-1
Pharmaceutical grade
Content: 99.5%
Appearance: white powder
Packing: 25 kg / cardboard drum can be split
Physical and chemical properties: white powder, odorless, taste slightly salty. Soluble in water, slightly soluble in ethanol, insoluble in ether.
Application: mainly used for urological angiography, but also for cardiovascular, cerebrovascular, peripheral blood vessels, bile duct and other angiography and a variety of injection method, such as joint cavity, fallopian tube, fistula and other angiography
-
CAS Registry Number:
737-31-5
-
Synonyms:
;Sodium diatrizoate dihydrate;sodium amidotrizoate;sodium 3,5-diacetamido-2,4,6-triiodobenzoate;3,5-Diacetamido-2,4,6-triiodobenzoic acid sodium salt~Diatrizonate sodium;Diatrizoate sodium;Sodium diatrizoate; Sodium diatrizoate dehydrate;3,5-Diacetylamino-2,4,6-trijodbenzosaeure natrium;Hypaque sodium;sodium 3,5-bis(acetylamino)-2,4,6-triiodobenzoate;sodium 3,5-bis(acetylamino)-2,4,6-triiodobenzoate dihydrate;
-
Molecular Formula:
C11H12I3N2NaO6
-
Molecular Weight:
671.926
-
Molecular Structure:
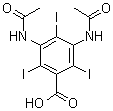
-
Hazard Symbols:
 Xn:Harmful;
Xn:Harmful;
-
Risk Codes:
R42/43:;
-
Safety Description:
S36:;
-
Company:
Hubei Yuancheng Pharmaceutical Co.,Ltd
[ China ]
-
Contact:
Kitty Hu
-
Tel:
027-88213979
-
Fax:
027-88608190-879
-
Email:
sales01@ycphar.com
Inquiry

Xn:Harmful;