Diclofenac Sodium
-
Post Date:
Oct 11,2018
-
Expiry Date:
Oct 11,2019
-
Detailed Description:
Cas No. :15307-79-6
Payment Method: T/T D/A D/P L/C
Diclofenac sodium
1. white or yellowish crystalline powder
2. Assay 99.9% min
3. BP, EP
4. GMP
Product Description
Raw material price of medicine Diclofenac sodium
1. white or yellowish crystalline powder
2. Assay 99.9% min
3. BP, EP
4. GMP
Item Standard Result
Characteristics A white or slightly yellowish crystalline powder White crystalline powder
Identificatiojn A. IR Accord
D. Test of sodium salt Conform
Appearance of solution 5.0% of methanol solution UV 440NM. NMT 0.05 0.009
Clarity of solution Clear Pass
Related substance Each specified impurity: NMT0.2% Not detected
Any unspecified impurity: NMT0.1% Not detected
Tatal impurities: NMT0.5% Not detected
Heavy metals NMT 10PPM Pass
Loss on drying NMT 0.5% 0.08%
Assay 99.0%~101.0% 99.98%
Conclusion: It accords with BP2012.
-
CAS Registry Number:
15307-79-6
-
Synonyms:
;Diclofenac sodium salt;2-[(2,6-dichlorophenyl)amino]benzeneacetic acid sodium salt;sodium [2-[(2,6-dichlorophenyl)amino]phenyl]acetate;2[(2,6-dichlorophenyl)amino]-benzeneacetic aci monosodium;{2-[(2,6-dichlorophenyl)amino]phenyl}acetic acid;sodium {2-[(2,6-dichlorophenyl)amino]phenyl}acetate;
-
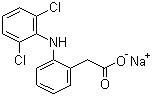
Molecular Formula:
C14H10Cl2NNaO2
-
Molecular Weight:
318.14
-
Molecular Structure:
-
Hazard Symbols:
 T:Toxic;
T:Toxic;
-
Risk Codes:
R25:;
-
Safety Description:
S22:;
S36/37:;
S45:;
Inquiry

T:Toxic;