Lansoprazole
-
Post Date:
Aug 06,2013
-
Expiry Date:
Feb 02,2014
-
Detailed Description:
Cas No. :103577-45-3
Specifications
1.99.0%
2.White to Off white crystalline powder
3.CAS NO.:103577-45-3
4.Treatment of duodenal ulcer
Product name
Lansoprazole
MFG. Date
20130322
Package
1kg/bag
Batch number
FH20130322
Testing Date
20130322
Exp Date
20150321
Standard
USP32
Quantity
200kg
Cas No.
103577-45-3
EINECS NO.
103577-45-3
Molecular Formula
C16H14F3N3O2S
Molecular Weight
369.37
Function
Main functions are as follows:
Treatment of duodenal ulcer
Applications
Main applications are as follows:
Medicine;Gastrointestinal Agents
Synonyms
Lansoprazole
Items Tested
Specification
Result
Characters
White to Off white crystalline powder
Confirms
Assay (HPLC)
99.0~101.0%
Confirms
Water
Not more than 0.5%
Confirms
Residue on ignition
Not more than 0.2%
Confirms
Single Impurity
Not more than 0.5%
Confirms
Total Impurity
Not more than 1.0%
Confirms
Heavy Metal
Not more than 20ppm
Confirms
Conclusion
Conforms USP32
-
CAS Registry Number:
103577-45-3
-
Synonyms:
;2-{[[3-methyl-4-(2,2,2-trifluoroethoxy)2-pyridinyl]methyl]sulfonyl}1H-benzimidazole;2-({[3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl]methyl}sulfinyl)-1H-benzimidazole;1-(1H-benzimidazol-2-yl)-2-[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridyl]ethanone;lansoprazile;
-
Molecular Formula:
C17H14F3N3O2
-
Molecular Weight:
349.3072
-
Molecular Structure:
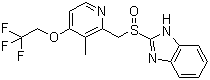
-
Hazard Symbols:
 Xi:Irritant;
Xi:Irritant;
-
Risk Codes:
R36/37/38:;
-
Safety Description:
S26:;
S36:;
-
Company:
Hubei MaxSource Chem Co., Ltd.
[ China ]
-
Contact:
Bale Yang
-
Tel:
+86-027-83555670
-
Fax:
862783555670
-
Email:
maxsource12@hotmail.com
Inquiry

Xi:Irritant;