Loxoprofen Sodium
-
Post Date:
Jan 02,2020
-
Expiry Date:
Jun 30,2020
-
Detailed Description:
Cas No. :80382-23-6
Quantity: 1Metric Tons
Payment Method: LC
The company mainly produces cardio-cerebrovascular, hypoglycemic, hypolipidemic and other pharmaceutical intermediates and APIs. The products produced by the company are mainly sold domestically and exported to overseas markets.
The workshops of the factory are all designed and constructed in accordance with EU and US FDA standards. The Wendeng Site covers an area of 1,800 acres, and has been put into production. The workshops are all designed and constructed in accordance with EU and US GMP standards.
-
CAS Registry Number:
80382-23-6
-
Synonyms:
;CS-600;Loxoprofen sodium dihydrate;Sodium 2-(4-(2-oxocyclopentylmethyl)phenyl)propionate dihydrate;alpha-Methyl-4-((2-oxocyclopentyl)methyl)benzeneacetate sodium salt dihydrate;Benzeneacetic acid, alpha-methyl-4-((2-oxocyclopentyl)methyl)-, sodium salt, dihydrate;sodium 2-{4-[(2-oxocyclopentyl)methyl]phenyl}propanoate hydrate (1:1:2);sodium 2-{4-[(2-oxocyclopentyl)methyl]phenyl}propanoate;LXP;
-
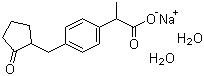
Molecular Formula:
C15H17NaO3
-
Molecular Weight:
268.2834
-
Molecular Structure:
-
Company:
Dijia Pharmaceutical Group Co., Ltd
-
Contact:
15066302597
-
Tel:
06313857109
-
Fax:
-
Email:
724419336@qq.com
Inquiry
