Pantoprazole Sodium USP standard
-
Post Date:
Dec 15,2023
-
Expiry Date:
Jun 12,2024
-
Detailed Description:
Cas No. :138786-67-1
Quantity: 100Kilograms
Specs:USP standard
Payment Method: T/T in advance
used for treatment of erosive esophagitis,white or almost white powder,USP standard
-
CAS Registry Number:
138786-67-1
-
Synonyms:
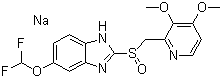
;Pantoprozole soclium Ph.Eur;PantoprozoleSodium;sodium 5-(difluoromethoxy)-2-{[(3,4-dimethoxypyridin-2-yl)methyl]sulfinyl}benzimidazol-1-ide;[5-(difluoromethoxy)-2-[(3,4-dimethoxy-2-pyridyl)methylsulfinyl]benzimidazol-1-yl]sodium;5-(Difluoromethoxy)-2-(((3,4-dimethoxy-2-pyridinyl)methyl) sulfinyl)-1H-benzimidazole sodium;
-
Molecular Formula:
C16H14F2N3NaO4S
-
Molecular Weight:
405.3516
-
Molecular Structure:
-
Company:
Hangzhou Maytime Bio-Tech Co.,Ltd. (Starview International Limited)
[ China ]
-
Contact:
Leo Tong
-
Tel:
+86-571-88925295,88920965
-
Fax:
+86-571-88218645
-
Email:
sales@maytime.com.cn
Inquiry
