Phenolphthalein CAS:77-09-8
-
Post Date:
Mar 06,2018
-
Expiry Date:
Mar 06,2019
-
Detailed Description:
Cas No. :77-09-8
Quantity: 1Kilograms
Specs:bodybuilding/ healthy care
Price:1 USD Kilograms
Payment Method: T/T Western union Moneygram
CAS:77-09-8
Mf:C20h14o4
MW:318.32
Melting point: 258-263 ° C
Density: 1.299
Flash point: 24 ° C
Solubility: <0.1 g/100 mL
Stability: Stable. Incompatible with strong oxidizing agents, alkalies.
NIST: Phenolphthalein(77-09-8)
EPA: 1(3H)-Isobenzofuranone, 3, 3-bis(4-hydroxyphenyl)-(77-09-8)
Risk category code: 40-22-10-36/38-23/25-11-36/37/38-68-62-45
Safety instruction: 45-36/37-33-24-16-7-36-26-53
RTECS: SM8380000
HS: 29322910
Poison material data: 77-09-8(Hazardous Substances Data)
Uses
Phenolphthalein has been used for over a century as a laxative, but is now being removed from over-the-counter laxatives because of concerns over carcinogenicity.Thymolphthalein is a related laxative made from thymol. See Phenolsulfonphthalein also.
Despite concerns regarding its carcinogenicity, the use of phenolphthalein as a laxative is unlikely to cause ovarian cancer. Phenolphthalein has been found to inhibit human cellular calcium influx via store-operated calcium entry (SOCE, see Calcium release activated channel § Structure). This is effected by its inhibiting thrombin and thapsigargin, two activators of SOCE that increase intracellular free calcium.
Phenolphthalein has been added to the European Chemicals Agency's candidate list for Substances of Very High Concern (SVHC).
A reduced form of phenolphthalein, phenolphthalin, which is colorless, is used in a test to identify substances thought to contain blood, commonly known as the Kastle-Meyer test. A dry sample is collected with a swab or filter paper. A few drops of alcohol, then a few drops of phenolphthalin, and finally a few drops of hydrogen peroxide are dripped onto the sample. If the sample contains hemoglobin, it will turn pink immediately upon addition of the peroxide, because of the generation of phenolphthalein. A positive test indicates the sample contains hemoglobin and, therefore, is likely blood. A false positive can result from the presence of substances with catalytic activity similar to heme. This test is not destructive to the sample; it can be kept and used in further tests. This test has the same reaction with blood from any animal, so further testing would be required to determine whether it originates from a human.
Description:
Phenolphthalein is a chemical compound with the formula C20H14O4 and is often written as "HIn" or "phph" in shorthand notation. Phenolphthalein is often used as an indicator in acid-base titrations. For this application, it turns colorless in acidic solutions and pink in basic solutions.
-
CAS Registry Number:
77-09-8
-
Synonyms:
;Phenolphthalein solution;3,3-bis(p-hydroxyphenyl)phthalide;,3-bis(4-hydroxyphenyl)-1(3H)-isobenzofuranore;3,3-bis(4-hydroxyphenyl)-2-benzofuran-1(3H)-one;2-[bis(4-hydroxyphenyl)methyl]benzoic acid;Phenolphthalien;
-
Molecular Formula:
C20H14O4
-
Molecular Weight:
318.3287
-
Molecular Structure:
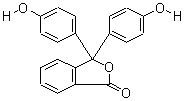
-
Hazard Symbols:
 Xn:Harmful;
Xn:Harmful;
-
Risk Codes:
R22:;
R40:;
-
Safety Description:
S36/37:;
S45:;
-
Company:
Wuhan Hezhong Bio-Chemical Manufature Co.,Ltd
[ China ]
-
Contact:
Laura
-
Tel:
18872220713
-
Fax:
86-27-88048077
-
Email:
uoyyphar@ycphar.com
Inquiry

Xn:Harmful;