Quality Antiestrogen Steroid Hormone Letrozoles Femara for Breast Cancer 112809-51-5
-
Post Date:
Jul 19,2017
-
Expiry Date:
Jan 15,2018
-
Detailed Description:
Cas No. :112809-51-5
Quantity: 500Kilograms
Price:2000 USD Kilograms
Letrozol is approved by the United States Food and Drug Administration (FDA) for the treatment of local or metastatic breast cancer that is hormone receptor positive or has an unknown receptor status in postmenopausal women.
Letrozol is intended for use only by women who are no longer of childbearing age. Possible candidates must have already ceased to menstruate. It is often used in patients who have already undergone other cancer treatments, such as radiation, chemotherapy, and used other cancer drugs, like tamoxifen. This nonsteroidal aromatase inhibitor may help prevent any remaining cancer cells from spreading.Femara is used for the treatment of post-menopause breast cancer.
Femara is a powerful Aromatase Inhibitor that was developed to fight breast cancer. For athletes and bodybuilders, it is a drug used to combat the estrogenic side effects of anabolic steroids waterretention, acne and gynocomastia. It will also raise testosterone levels because of the lowered estrogen in the body. Side effects from include a lowered sex and continuous use can lead to lowered lipid function and an impaired immune system.
-
CAS Registry Number:
112809-51-5
-
Synonyms:
;4,4'-(1h-1,2,4-Triazol-1-Ylmethylene)Bisbenzonitrile;Lelrozol;4,4'-(1h-1,2,4-Triazol-1-Ylmethylene) Bis-Benzonitrile;Femara;Letrozol;Letrazole;
-
Molecular Formula:
C17H11N5
-
Molecular Weight:
285.31
-
Molecular Structure:
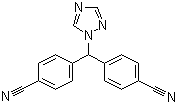
-
Company:
HongKong Blue Universal Co., Limited.
[ China ]
-
Contact:
Andrew George
-
Tel:
+86-170-76116742
-
Fax:
86-170-76116742
-
Email:
fxhandrew@outlook.com
Inquiry
