Roflumilast 162401-32-3
-
Post Date:
Mar 29,2025
-
Expiry Date:
Mar 29,2026
-
Detailed Description:
Cas No. :162401-32-3
Quantity: 100Kilograms
Specs:Purity (HPLC) ≥99.0%
Payment Method: L/C; T/T
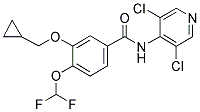
Roflumilast
Chemical name: 3-(Cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl)-4-(difluoromethoxy)benzamide
CAS No.: 162401-32-3
Molecular Formula: C17H14Cl2F2N2O3
Molecular Weight: 403.21
Function: On March 1,2011, the FDA approved the chronic obstructive pneumonia drug Daliresp (Roflumilast), the drug used daily, can reduce asthma attack frequency or mitigate serious chronic obstructive pneumonia (COPD) symptoms. Roflumilast is a new category of COPD drug, phosphodiesterase - 4 (PDE - 4) inhibitors. Can be used among patients with serious COPD cough and bronchial symptom related much sputum, does not apply to mild emphysema.
Items Specification
Appearance White or off white crystalline powder
Loss on drying ≤0. 5%
Single impurity ≤0.50%
Purity (HPLC) ≥99.0%
Packing Pack in two plastic bags inside and Alu-foil bag outside.
Storage Store in a well-closed place with room temperature and no direct sun light.
Shelf life Three years when properly stored.
Free Radiation Effect This product is not been irradiated.
-
CAS Registry Number:
162401-32-3
-
Synonyms:
;3-(Cyclopropylmethoxy)-N-(3,5-dichloro-4-pyridinyl)-4-(difluoromethoxy)benzamide;B 9302-107;BY 217;BYK 20869;Daxas;3-(Cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl)-4-(difluoromethoxy)benzamide;N-(cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl)-4-(difluoromethoxy)benzamide;
-
Molecular Formula:
C17H14Cl2F2N2O3
-
Molecular Weight:
403.2075
-
Molecular Structure:
Inquiry
