Sodium Hyaluronate
-
Post Date:
Mar 23,2021 -
Expiry Date:
Sep 19,2021 -
Detailed Description:
Cas No. :9067-32-7Pharmaceutical Grade of HA
Test Items
Eyes-drop Grade
Injection Grade
Description
White, fine powder
White, fine powder
pH
6.0-7.5
6.0-7.5
Assay
≥91.0%
≥91.0%
Transparency
≥99.0%
≥99.0%
Molecular Weight
≥1.00×10 6
≥2.00×10 6
Glucuronic Acid
≥44.0%
≥44.0%
Protein
≤0.1%
≤0.1%
Heavy Metals
≤20ppm
≤20ppm
Arsenic
≤2ppm
≤2ppm
Loss on Drying
≤10.0%
≤10.0%
Nitrogen
3.0-4.0%
3.0-4.0%
Intrinsic Viscosity
≥18.0dL/g
≥30.0dL/g
Bacterial Endotoxin
≤0.5IU/mg
≤0.05IU/mg
Bacteria Count
<10cfu/g
<10cfu/g
Mold and Yeast
<10cfu/g
<10cfu/g
Staphylococcus Aureus
Negative
Negative
Pseudomonas Aeruginosa
Negative
Negative
-
CAS Registry Number:
9067-32-7 -
Synonyms:
;Hyaluronic acid sodium salt;HA Sodium;Hyaluronate Sodium;Oligomerie Sodium Hysluronate; -
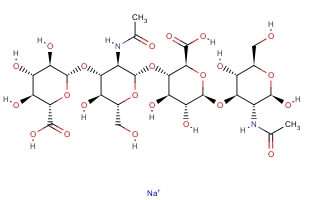
Molecular Formula:
C28H44N2O23·Na -
Molecular Weight:
799.6366 -
Molecular Structure:
-
Safety Description:
S22:;
S24/25:;
-
Company:
Shandong luning pharmaceutical co., Ltd., [ China ] -
Contact:
Mr.Yang -
Tel:
0086-546-7720105 -
Fax:
0086-546-7793666 -
Email:
572182191@qq.com