Sodium acetate
-
Post Date:
Jul 23,2016 -
Expiry Date:
Aug 22,2016 -
Detailed Description:
Cas No. :127-09-3 Quantity: 1000Metric Tons
Specs:white powder
Product Sodium Acetate CAS 127-09-3(Anhydrous sodium
acetate)6131-90-4
(Sodium acetate trihydrate)INS 262(i) Molecular formula C2H3NaO2; C2H3NaO2·3H2O Quality
SpecificationStandard Item The Ministry
of Health
announcement
specified
standard of
2011 19 -07BP2012 FCCV USP35 EP7.0 Content(dry
basis)≥98.5% 99.0% ~
101.0 %
(dried
substance).99%~
101%99%~101% 99%~101% Reducing
substances---------- passed test ---------- ---------- passed test Appearance of
solution---------- clear and
colourless---------- Insoluble
matter
≤0.05%clear and
colourlessFree acid and
free basepassed test pH=7.5 ~ 9.0 ---------- pH=7.5 ~ 9.2 pH=7.5 ~ 9.0 Heavy metals
(asPb)≤10mg/Kg ≤ 2
mg /kg---------- ---------- Chlorine ---------- ≤200ppm ---------- ≤0.035% ≤200ppm Sulfate ---------- ≤200ppm ---------- ≤0.005% ≤200ppm Aluminum salts ---------- ≤0.2ppm ---------- ≤0.2 µg /g ≤0.2ppm Calcium and
magnesium ions---------- ≤50ppm ---------- pass test ≤50ppm Heavy metals ---------- ≤10ppm ---------- ≤0.001% ≤10ppm Fe ---------- ≤10ppm ---------- ---------- ≤10ppm As(as As) ≤3mg/Kg ≤2ppm ---------- ---------- ≤2ppm Loss on drying
(crystalline
products)36.0~42.0% 39.0%-40.5% 36.0% ~
41.0%38.0% ~41.0% 39.0 %~ 40.5 % Loss on drying
(anhydrous
product)≤2% ---------- ≤ 1.0% ≤ 1.0% ---------- Alkalinity ---------- ---------- Anhydrous:
≤0.2%;
Trihydrate:
≤0.05%---------- ---------- Properties:The crystalline product is colorless transparent crystals or white crystalline powder, anhydrous
product is white crystalline powder or lumps.Odorless. Shape of our product is white powder,
spherical or cylindrical particles.Heat and light stabilizers.Hygroscopic, soluble in water (40%).Application
1.Used for the determination of lead, zinc, aluminum, iron, cobalt, nickel, tin, antimony.Complexation
stabilizer,adjuvant of acetylation,desiccant,mordant.Used as the esterifying agent in organic
synthesis and inphotography drugs, pharmaceuticals, printing and dyeing mordant, buffer, chemical
reagent, meat preservation, pigments, leather tanning and many other aspects.
2.Used as buffers, flavoring agents and pH adjusting agents.As a buffer of the flavoring agent,it can
ease the bad smell and prevent discoloration when using 1% -3%,also can possess anti-enzyme effect
,when using 0.1%~0.3% in fish surimi products and bread.Used as sour agent of sauces、sauerkraut、
mayonnaise、kamaboko、sausage、bread、rice cake,etc.when mixed with methylcellulose and
phosphate to increase the shelf life of sausage, bread and rice cake.
3.Used as anti-scorcher in sulfer regulated chloroprene rubber coking ,the amount is about 0.5 phr
.Also can be used as the crosslinking agent of animal glue.Dosage In accordance with FDA § 184.1 (b) (1), the highest limit:Breakfast cereal,0.007%;Grease,0.5%;
Cereals and pasta, snack foods,0.6%;Hard Candy,0.15%;Jams and jellies, meat products,
0.12%;Fudge,0.2%;Soups and soup premix, sweet sauce,0.05%。
In accordance with GB2760-2011,the maximum amount in the complex condiment :10g/kg;In puffed
food:1g/kg.Packaging Net weight: 25kg,paper-plastic bag or woven bag lined with PE bag,can also be customized according to
customer‘s requirements of packaging.Storage Stored in cool, dry and ventilated place.Must not be loaded and transported with toxic, hazardous and
polluting substances.
-
CAS Registry Number:
127-09-3 -
Synonyms:
;sodium ethanoate;Acetic acid sodium salt anhydrous;Sodium Acetate, Anhydrous;Anhydrous sodium acetate;Sodium acetate;Acetic acid, sodium salt;Acetic acid sodium salt;acetate sodium anhydrous;Sodium Acetate,Anhydrous; -
Molecular Formula:
C2H3NaO2 -
Molecular Weight:
82.03 -
Molecular Structure:
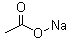
-
Safety Description:
S24/25:;
-
Company:
Tengzhou Zhongzheng Chemical Co., Ltd. [ China ] -
Contact:
Mr Long -
Tel:
+86-632-5956199 5567819 -
Fax:
+86-632-5561919 -
Email:
sales@zhongzhengchem.com