Hyaluronidase
111Category: Intermediates/Pharmaceutical intermediates
CAS NO: 9067-32-7 EC NO: Molecular Formula: C28H44N2O23��Na Molecular Weight: 799.6366 Specification: Not less than 500 iu/mg InChI: InChI=1/C28H44N2O23.Na/c1-5(33)29-9-18(11(35)7(3-31)47-25(9)46)49-28-17(41)15(39)20(22(53-28)24(44)45)51-26-10(30-6(2)34)19(12(36)8(4-32)48-26)50-27-16(40)13(37)14(38)21(52-27)23(42)43;/h7-22,25-28,31-32,35-41,46H,3-4H2,1-2H3,(H,29,33)(H,30,34)(H,42,43)(H,44,45);/q;+1/t7-,8-,9-,10-,11-,12-,13+,14+,15-,16-,17-,18-,19-,20+,21+,22+,25-,26+,27-,28-;/m1./s1 Packing: bag/tin Product description:
Testing Items (EP 6.0& USP 32) Standard Specification Description White or light yellow powder Identification Should Comply PH Value 6.4-7.4 Appearance of solution Should Clear Bacterial endotoxins Not more than 2.30 endotoxin units per USP units of Hyaluronidase Solubllity(100mg/ml) Soluble in water, practically insoluble in acetone, in ethanol(95%) and in ether Limit of yrosine Not more than 0.25 μg per USP units of Hyaluronidase Loss on drying Not more than 5.0% Potency Not less than 500 iu/mg Conclusion This Product complies with EP 6.0 & USP32Uses: OPHTHALMIC GRADE Synonyms: Hyaluronic acid sodium salt;HA Sodium;Hyaluronate Sodium;Oligomerie Sodium Hysluronate; Molecular Structure: 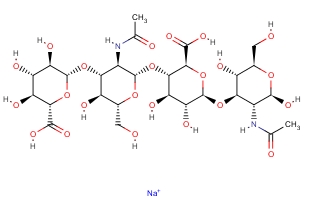
if you are sourcing Hyaluronidase from China ,just feel free to inquire




