 CN
China Suppliers > Weishi >
CN
China Suppliers > Weishi > year
| Category: | Pharmaceuticals and Biochemicals/Vitamin, amino acids and coenzymes |
|
|
| CAS NO: | 67-97-0 | ||
| EC NO: | 200-673-2 | ||
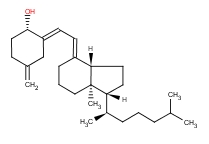
| Molecular Formula: | C27H44O | ||
| Molecular Weight: | 384.6377 | ||
| Specification: | |||
| InChI: | InChI=1/C27H44O/c1-19(2)8-6-9-21(4)25-15-16-26-22(10-7-17-27(25,26)5)12-13-23-18-24(28)14-11-20(23)3/h12-13,19,21,24-26,28H,3,6-11,14-18H2,1-2,4-5H3/b22-12+,23-13- | ||
| Packing: | 5-25kg fibreboard box with AL-PE laminated liner, heat-sealed | ||
| Product description: BHT stab Chemical names Cholecalciferol CAS No. 67-97-0 EINECS No. 200-673-2 Description Free-flowing off-white powder consisting of fine spherical particles. Composition The powder particles contain vitamin D3 in medium chain triglycerides in droplets of 1-2μm embedded in a matrix of gelatin and sucrose and coated with modified starch. The product contains t-butylhyd roxytoluene(BHT,E 321)and DL-a-tocopherol(E307) as an antioxidant. Solubility The product can be dispersed in warm water (35-40℃)to form a milky emulsion. Practically no vitamin D can be extracted with ether form the powder. Specification Assay;min.850,000I.U./g (=21,250μg cholecalciferol/g) Loss on drying:≤5%(4 hours at 105℃) Monographs The vitamin complies with the current USP and Ph.Eur.monographs. Particle-size distribution 100%smaller than 425μm(40mesh USP) min. 90% smaller than 250μm(60mesh USP) max.15% smaller than 150μm(100mesh USP) Bulk density Approx.0.6g/ml Stabilization/stability The product is stabilized with BHT(E321). The stability of the vitamin D3 in the dry powder is very good even in the presence of minerals. The product has good resistance to pressure and very little vitamin D3 is expressed during tabletting so that it exhibits good stability in tablets. The product is stable for at least 30 months when it is stored in the original containers room temperature(about 25℃). Packaging 5-25kg fibreboard box with AL-PE laminated liner, heat-sealed. Storage The product should be stored in the original containers at room temperature(about 25℃). Applications Pharmaceutical preparations: Because of its excellent tabletting and flow properties, the product is suitable both for direct compression(tablets with vitamin D3 as the sole active ingredient, multivitamin tablets, multivitamin/mineral tablets etc.) and for the manufacture of hard gelatin capsules. Note Dry vitamin D3 850 must be handled in accordance with the safety data sheet. |
|||
| Synonyms: | Cholecalciferol [USAN:BAN:JAN];Cholecalciferol;9,10-Secocholestra-5,7,10(19)-trien-3-ol, (3beta,5Z,7E)-;7-Dehydrocholesterol, irradiated;7-Dehydrocholestrol, activated;9,10-Seco(5Z,7E)-5,7,10(19)-cholestatrien-3-ol;9,10-Secocholesta-5,7,10(19)-trien-3-beta-ol;Activated 7-dehydrocholesterol;Arachitol;Colecalciferol;Colecalciferolo;Colecalciferolo [DCIT];Colecalciferolum;Colecalciferolum [INN-Latin];Colecalcipherol;D3-Vicotrat;D3-Vigantol;Delsterol;Deparal;Duphafral D3 1000;EPA Pesticide Chemical Code 202901;Ebivit;HSDB 820;Irradiated 7-dehydrocholesterol;NEO Dohyfral D3;NSC 375571;Oleovitamin D3;Quintox;Rampage;Ricketon;Trivitan;Vi-de-3-hydrosol;Vigantol;Vigorsan;Vitinc dan-dee-3;9,10-Secocholesta-5,7,10(19)-trien-3-ol, (3beta,5Z,7E)-;Cyclohexanol, 3-((2E)-2-((1R,3aS,7aR)-1-((1R)-1,5-dimethylhexyl)octahydro-7a-methyl-4H-inden-4-ylidene)ethylidene)-4-methylene-, (1S,3Z)-;(3S,5Z,7E)-9,10-secocholesta-5,7,10-trien-3-ol;(5Z,7E)-9,10-secocholesta-5,7,10-trien-3-ol; | ||
| Molecular Structure: |
 |
||